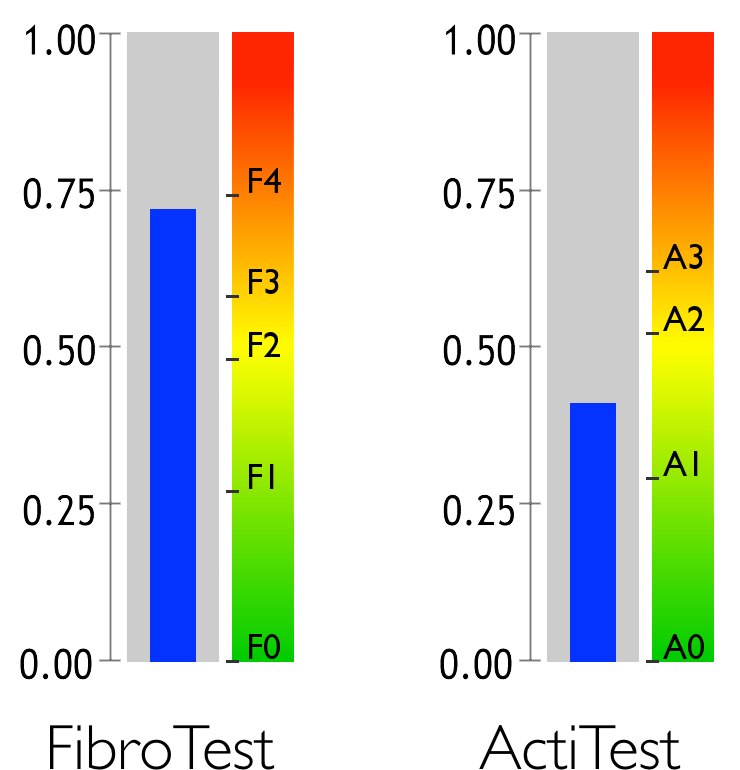
Fibrosis and activity
FibroTest-ActiTest estimates the levels of fibrosis/cirrhosis in the liver and the level of necroinflammatory activity.
Fibrosis and activity are the two main causes of liver disease.
Fibrosis is a medical condition caused by the reaction of a diseased liver. Hepatic fibrosis is typically compared to a form of scar tissue that progresses throughout the liver. The most serious stage of fibrosis is known as cirrhosis.
Activity is liver inflammation caused by disease. It is often compared to a burn.
FibroTest is recommended by WHO, the American Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL) and the Asia-Pacific Association for the Study of the Liver (APASL) for evaluating hepatic fibrosis in chronic hepatitis C patients.
FibroTest is recommended EASL-ALEH, APASL and WHO for diagnosing fibrosis in carriers of chronic hepatitis B virus.
FibroTest is recommended by the EASL-ALEH for co-infected HIV carriers.
FibroTest is also used to provide access to new-generation non-interferon treatment for hepatitis C.
FibroTest is approved and recommended by the European Associations for the Study of the Liver (EASL), Diabetes (EASD) and Obesity (EASO) for the purpose of evaluating fibrosis in patients suffering from metabolic conditions (NAFLD) and by EASL when evaluating patients who consume excess alcohol.
The reference test
FibroTest is protected by an international patent and is founded on hundreds of scientific publications.
FibroTest is a non-invasive test that is applicable to the largest number of patients (98%) as well as being the most reliable .
FibroTest offers the best performance of any test, at all stages of fibrosis: from a healthy liver to cirrhosis.
Out of all non-invasive tests, FibroTest is the least sensitive to known risk factors for false positives and false negatives, such asnecroinflammatory activity and steatosis, and it is not operator-dependent.
| FibroTest | Transient Elastography | APRI | FIB-4 | |
|---|---|---|---|---|
| Applicability | ||||
| Performance from F0 to F3 | ||||
| Performance for F4 | ||||
| False positive due to inflammation | ||||
| Cost | ||||
| Prognostic |
6 components, 2 scores
FibroTest combines five standard biomarkers
- Gamma-GT
- Total bilirubin
- Alpha-2-macroglobulin
- Apolipoprotein A1
- Haptoglobin
ActiTest adds a direct marker for inflammatory activity:
- Alanine aminotransferase (ALT)
These markers are weighted depending on the patient’s age and sex.
Tests may take place in any laboratory that is compliant with technical recommendations.
There is no need to fast to have FibroTest-ActiTest.
FibroMax
FibroTest and ActiTest are also included in FibroMax™, which offers a more complete picture of the condition of the liver.
References
- Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection book 2014;None:None.
реферат - Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015;62:3.
реферат
статья - EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015;63:1.
реферат
статья - Shiha G, Sarin SK, Ibrahim AE, Omata M, Kumar A, Lesmana LA, Leung N, Tozun N, Hamid S, Jafri W, Maruyama H, Bedossa P, Pinzani M, Chawla Y, Esmat G, Doss W, Elzanaty T, Sakhuja P, Nasr AM, Omar A, Wai CT, Abdallah A, Salama M, Hamed A, Yousry A, Waked I, Elsahar M, Fateen A, Mogawer S, Hamdy H, Elwakil R. Liver fibrosis: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol Int 2009;3:2.
реферат
статья - Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis B Infection book 2015;None:None.
реферат - Munteanu M, Tiniakos D, Anstee Q, Charlotte F, Marchesini G, Bugianesi E, Trauner M, Romero Gomez M, Oliveira C, Day C, Dufour JF, Bellentani S, Ngo Y, Traussnig S, Perazzo H, Deckmyn O, Bedossa P, Ratziu V, Poynard T. Diagnostic performance of FibroTest, SteatoTest and ActiTest in patients with NAFLD using the SAF score as histological reference. Aliment. Pharmacol. Ther. 2016;44:8.
реферат
статья - EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes Facts 2016;9:2.
реферат
статья - EASL clinical practical guidelines: management of alcoholic liver disease. J. Hepatol. 2012;57:2.
реферат
статья - Poynard T, Munteanu M, Deckmyn O, Ngo Y, Drane F, Messous D, Castille JM, Housset C, Ratziu V, Imbert-Bismut F. Applicability and precautions of use of liver injury biomarker FibroTest. A reappraisal at 7 years of age. BMC Gastroenterol 2011;11:None.
реферат
статья - Houot M, Ngo Y, Munteanu M, Marque S, Poynard T. Systematic review with meta-analysis: direct comparisons of biomarkers for the diagnosis of fibrosis in chronic hepatitis C and B. Aliment. Pharmacol. Ther. 2016;43:1.
реферат
статья - Poynard T, De Lédinghen V, Zarski JP, Stanciu C, Munteanu M, Vergniol J, France J, Trifan A, Lenaour G, Vaillant JC, Ratziu V, Charlotte F. Performances of Elasto-FibroTest(®), a combination between FibroTest(®) and liver stiffness measurements for assessing the stage of liver fibrosis in patients with chronic hepatitis C. Clin Res Hepatol Gastroenterol 2012;36:5.
реферат
статья