FibroMax™ offers an end-to-end liver diagnostic service from a single blood sample.
Five main diagnostic tests are included in FibroMax, for a complete assessment of the condition of the liver and the five main causes of liver disease.
FibroMax = FibroTest + ActiTest + SteatoTest + NashTest + AshTest
Metabolic diseases, steatosis
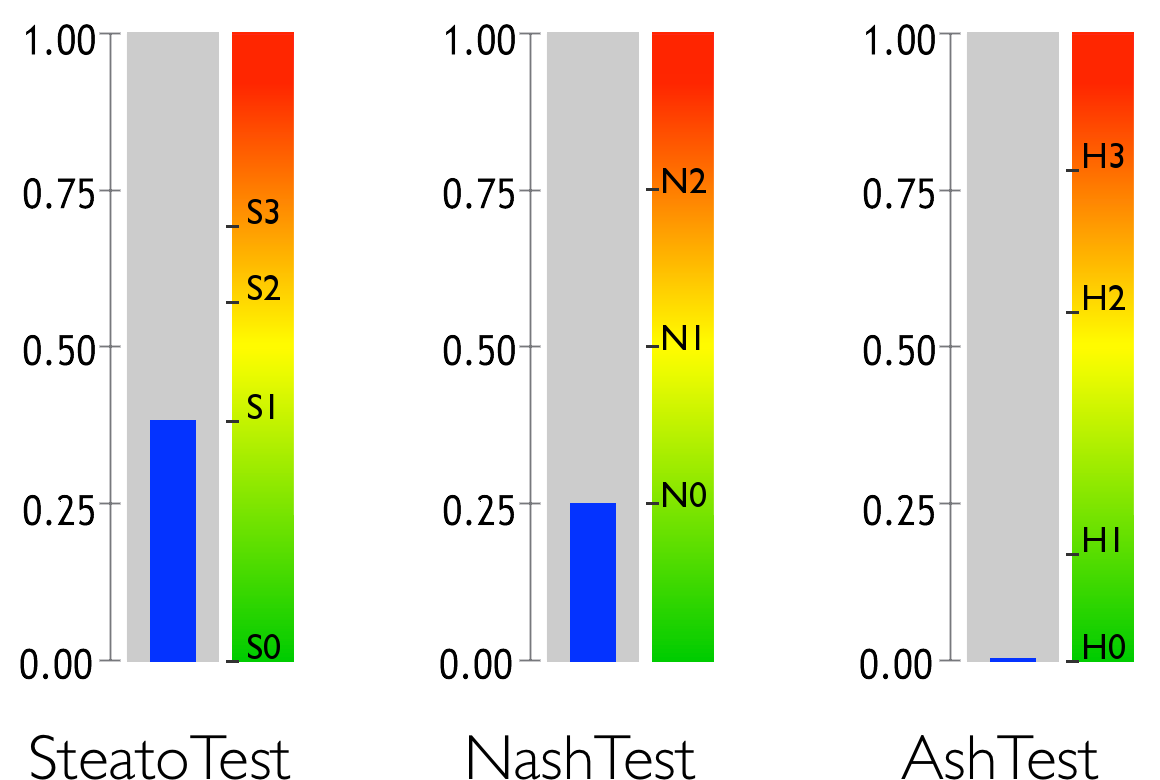
Hepatic steatosis, which is assessed by the SteatoTest, is a build-up of fat in the liver, which frequently causes elevated levels of Gamma-GT and transaminase.
Non-alcoholic steatohepatitis (NASH)is an inflammatory disease of the liver which is caused by metabolic conditions including excess weight, arterial hypertension (high blood pressure) and abnormal levels of triglycerides or cholesterol. The NashTest evaluates the level of necroinflammatory activity caused by the metabolic condition.
Alcoholic steato-hepatitis (ASH) – is an inflammatory disease of the liver caused by excessive alcohol consumption. The AshTest evaluates the level of this necroinflammatory activity due to alcohol.
Fibrosis and activity
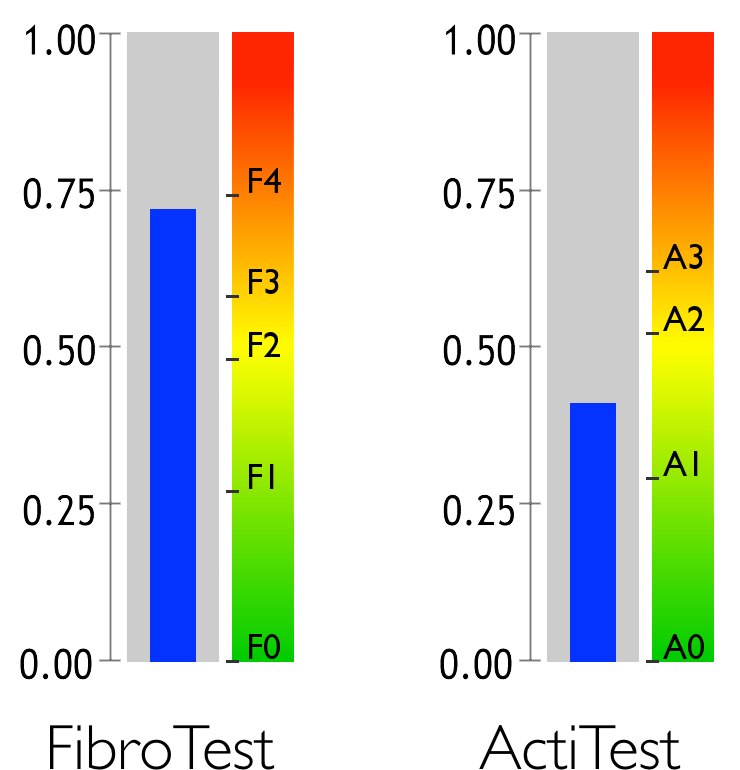
FibroTest estimates the levels of hepatic fibrosis and cirrhosis, while ActiTest estimates the level of activity.
Fibrosis and necroinflammatory activity are the two main causes of liver disease.
Fibrosis is a medical condition caused by the reaction of a diseased liver. Hepatic fibrosis is typically compared to a form of scar tissue that progresses throughout the liver. The most serious stage of fibrosis is known as cirrhosis.
Activity refers to the level of liver inflammation caused by disease. It is often compared to a burn.
Highly accurate tests
FibroTest
FibroTest is recommended by WHO, the american Association for the Study of Liver Diseases (AASLD), the European Association for the Study of the Liver (EASL), and the Asia-Pacific Association for the Study of the Liver (APASL) 5for testing for hepatic fibrosis in chronic hepatitis C patients, with or without HIV co-infections, as well as patients with metabolic conditions or who consume excess alcohol.
FibroTest is widely used to give access to non-interferon treatments for combating the hepatitis C virus and for patient monitoring.
FibroTest, when combined with ActiTest, makes it possible to identify asymptomatic carriers of the hepatitis B virus as well as potential treatments.
FibroTest is specifically designed for cirrhosis and approved for the purposes of classifying its severity into one of three classes.
FibroTest is the only test that is capable of classifying the early stages of fibrosis.
FibroTest can be used for longitudinal monitoring of patients with chronic liver disease.
ActiTest
ActiTest is superior to ALT, which is the standard biomarker for necroinflammatory activity.
ActiTest and FibroTest make it possible to identify asymptomatic carriers of hepatitis B.
ActiTest and FibroTest make it possible to identify potential treatments and monitor the progression of chronic viral hepatitis.
ActiTest is a quantitative biomarker which has been validated in subjects who are at high metabolic risk, whether or not this is accompanied by severe obesity.
SteatoTest
SteatoTest estimates the degree of steatosis in subjects at high metabolic risk, patients who consume excess alcohol or chronic carriers of the hepatitis B or C viruses.
As a quantitative biomarker for steatosis, SteatoTest allows longitudinal monitoring to be performed on patients.
SteatoTest is approved as a predictor of cardiovascular risk associated with steatosis.
NashTest
NashTest acts as a reliable predictor of the presence or absence of NASH.
NashTest, when combined with FibroTest, has demonstrated its value in screening for NASH in patients with metabolic risk factors.
AshTest
AshTest – is a fast alternative to transjugular hepatic biopsies, which therefore makes it possible to treat acute alcoholic steatohepatitis (ASH) in patients suffering from alcohol-related liver disease.
10 components, 5 scores
FibroMax combines ten standard biomarkers:
- Gamma-GT
- Total bilirubin
- Alpha-2-macroglobulin
- Apolipoprotein A1
- Haptoglobin
- Alanine aminotransferase (ALT)
- AST Transaminase
- Triglycerides
- Cholesterol
- Fasting glucose
These markers are weighted depending on the patient’s age, sex, weight, and height.
FibroMax tests must be done on an empty stomach in any local medical test laboratory that complies with technical recommendations.
References
- Morra R, Munteanu M, Imbert-Bismut F, Messous D, Ratziu V, Poynard T. FibroMAX: towards a new universal biomarker of liver disease? Expert Rev. Mol. Diagn. 2007;7:5.
реферат
статья - . Guidelines for the Screening, Care and Treatment of Persons with Hepatitis C Infection book 2014;None:None.
реферат - . Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015;62:3.
реферат
статья - . EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J. Hepatol. 2015;63:1.
реферат
статья - Shiha G, Sarin SK, Ibrahim AE, Omata M, Kumar A, Lesmana LA, Leung N, Tozun N, Hamid S, Jafri W, Maruyama H, Bedossa P, Pinzani M, Chawla Y, Esmat G, Doss W, Elzanaty T, Sakhuja P, Nasr AM, Omar A, Wai CT, Abdallah A, Salama M, Hamed A, Yousry A, Waked I, Elsahar M, Fateen A, Mogawer S, Hamdy H, Elwakil R. Liver fibrosis: consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL). Hepatol Int 2009;3:2.
реферат
статья - . EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes Facts 2016;9:2.
реферат
статья - Munteanu M, Tiniakos D, Anstee Q, Charlotte F, Marchesini G, Bugianesi E, Trauner M, Romero Gomez M, Oliveira C, Day C, Dufour JF, Bellentani S, Ngo Y, Traussnig S, Perazzo H, Deckmyn O, Bedossa P, Ratziu V, Poynard T. Diagnostic performance of FibroTest, SteatoTest and ActiTest in patients with NAFLD using the SAF score as histological reference. Aliment. Pharmacol. Ther. 2016;44:8.
реферат
статья - . EASL clinical practical guidelines: management of alcoholic liver disease. J. Hepatol. 2012;57:2.
реферат
статья - Poynard T, Ngo Y, Munteanu M, Thabut D, Massard J, Moussalli J, Varaud A, Benhamou Y, Ratziu V. Biomarkers of liver injury for hepatitis clinical trials: a meta-analysis of longitudinal studies. Antivir. Ther. (Lond.) 2010;15:4.
реферат
статья - Poynard T, Vergniol J, Ngo Y, Foucher J, Thibault V, Munteanu M, Merrouche W, Lebray P, Rudler M, Deckmyn O, Perazzo H, Thabut D, Ratziu V, De Lédinghen V. Staging chronic hepatitis B into seven categories, defining inactive carriers and assessing treatment impact using a fibrosis biomarker (FibroTest®) and elastography (FibroScan®). J. Hepatol. 2014;61:5.
реферат
статья - Ratziu V, De Lédinghen V, Oberti F, Mathurin P, Wartelle-Bladou C, Renou C, Sogni P, Maynard M, Larrey D, Serfaty L, Bonnefont-Rousselot D, Bastard JP, Rivière M, Spénard J. A randomized controlled trial of high-dose ursodesoxycholic acid for nonalcoholic steatohepatitis. J. Hepatol. 2011;54:5.
реферат
статья - Lassailly G, Caiazzo R, Hollebecque A, Buob D, Leteurtre E, Arnalsteen L, Louvet A, Pigeyre M, Raverdy V, Verkindt H, Six MF, Eberle C, Patrice A, Dharancy S, Romon M, Pattou F, Mathurin P. Validation of noninvasive biomarkers (FibroTest, SteatoTest, and NashTest) for prediction of liver injury in patients with morbid obesity. Eur J Gastroenterol Hepatol 2011;23:6.
реферат
статья - Poynard T, Munteanu M, Ngo Y, Castéra L, Halfon P, Ratziu V, Imbert-Bismut F, Thabut D, Bourliere M, Cacoub P, Messous D, De Lédinghen V. ActiTest accuracy for the assessment of histological activity grades in patients with chronic hepatitis C, an overview using Obuchowski measure. Gastroenterol. Clin. Biol. 2010;34:6-7.
реферат
статья - Poynard T, Lassailly G, Diaz E, Clement K, Caiazzo R, Tordjman J, Munteanu M, Perazzo H, Demol B, Callafe R, Pattou F, Charlotte F, Bedossa P, Mathurin P, Ratziu V. Performance of biomarkers FibroTest, ActiTest, SteatoTest, and NashTest in patients with severe obesity: meta analysis of individual patient data. PLoS ONE 2012;7:3.
реферат
статья - Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, Capron D, Abella A, Massard J, Ngo Y, Munteanu M, Mercadier A, Manns M, Albrecht J. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol 2005;4:None.
реферат
статья - Supronowicz Ł, Wójtowicz E, Cylwik B, Gruszewska E, Chrostek L. [The diagnostic value of non-invasive biochemical biomarkers in alcohol abuse]. Pol. Merkur. Lekarski 2013;35:207.
реферат - Gudowska M, Wójtowicz E, Cylwik B, Gruszewska E, Chrostek L. The Distribution of Liver Steatosis, Fibrosis, Steatohepatitis and Inflammation Activity in Alcoholics According to FibroMax Test. Adv Clin Exp Med 2015;24:5.
реферат
статья - Poynard T, Vergniol J, Ngo Y, Foucher J, Munteanu M, Merrouche W, Colombo M, Thibault V, Schiff E, Brass CA, Albrecht JK, Rudler M, Deckmyn O, Lebray P, Thabut D, Ratziu V, De Lédinghen V. Staging chronic hepatitis C in seven categories using fibrosis biomarker (FibroTest™) and transient elastography (FibroScan®). J. Hepatol. 2014;60:4.
реферат
статья - Munteanu M, Houot M, Ngo Y, Poynard T. Biopsy as well as FibroTest/Fibrosure is suboptimal for discriminating intermediate fibrosis stages in patients with chronic hepatitis B. Am. J. Gastroenterol. 2014;109:8.
реферат
статья - Perazzo H, Munteanu M, Ngo Y, Lebray P, Seurat N, Rutka F, Couteau M, Jacqueminet S, Giral P, Monneret D, Imbert-Bismut F, Ratziu V, Hartemann-Huertier A, Housset C, Poynard T. Prognostic value of liver fibrosis and steatosis biomarkers in type-2 diabetes and dyslipidaemia. Aliment. Pharmacol. Ther. 2014;40:9.
реферат
статья - Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F, Massard J, Bonyhay L, Tahiri M, Thabut D, Cadranel JF, Le Bail B, De Lédinghen V. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 2006;6:None.
реферат
статья - Ratziu V, Giral P, Munteanu M, Messous D, Mercadier A, Bernard M, Morra R, Imbert-Bismut F, Bruckert E, Poynard T. Screening for liver disease using non-invasive biomarkers (FibroTest, SteatoTest and NashTest) in patients with hyperlipidaemia. Aliment. Pharmacol. Ther. 2007;25:2.
реферат
статья - Zelber-Sagi S, Nitzan-Kaluski D, Goldsmith R, Webb M, Zvibel I, Goldiner I, Blendis L, Halpern Z, Oren R. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology 2008;48:6.
реферат
статья - Zelber-Sagi S, Salomone F, Webb M, Lotan R, Yeshua H, Halpern Z, Santo E, Oren R, Shibolet O. Coffee consumption and nonalcoholic fatty liver onset: a prospective study in the general population. Transl Res 2015;165:3.
реферат
статья - Rudler M, Mouri S, Charlotte F, Cluzel P, Ngo Y, Munteanu M, Lebray P, Ratziu V, Thabut D, Poynard T. Validation of AshTest as a Non-Invasive Alternative to Transjugular Liver Biopsy in Patients with Suspected Severe Acute Alcoholic Hepatitis. PLoS ONE 2015;10:8.
реферат
статья - Thabut D, Naveau S, Charlotte F, Massard J, Ratziu V, Imbert-Bismut F, Cazals-Hatem D, Abella A, Messous D, Beuzen F, Munteanu M, Taieb J, Moreau R, Lebrec D, Poynard T. The diagnostic value of biomarkers (AshTest) for the prediction of alcoholic steato-hepatitis in patients with chronic alcoholic liver disease. J. Hepatol. 2006;44:6.
реферат
статья